Abstract
A multiple dose regimen is a concept that is used for certain diseases as arthritis, heart diseases etc. It is used to increase the therapeutic activity and clinical efficacy of the drugs. Drugs are usually used in single doses to produce acute effect but in cases where chronic affect is required multiple dosage regimen is required. Designing the dosage regimen and maintaining the drug concentration in blood plasma after administration are the primary goals to achieve safety and efficacy. That is to maintain drug concentration in therapeutic window. Moreover, the IV infusion and continuous IV diffusion is reviewed.
Key Words
Multiple Dose Regimen, Loading Dose, Drug Accumulation, IV Infusion, Pharmacokinetics
Introduction
With the advancement in science and technology new procedures are being introduced to improve the living standards. Amini, M. Reis, M. Wide-Swensson, D. A. (2020)
Dosing frequency and required dose are determined by the pathway a drug takes when administered. To obtain the optimal effect of the therapy the multiple dosage regimen is used. Multiple dosage regimen can be defined as the administration of the drug substance in appropriate doses and at appropriate time intervals in required dose to maintain the concentration of drug in plasma throughout the entire therapy period drug remains in the therapeutic window. Pharmacokinetic parameters of a drug remains constant during the entire course of the therapy after the proper dosage regimen is established. One compartment model calculation is also applicable to the two compartment models.
• B
• Ke
• Vd
• ss
• Drug Administration at fixed dose and fixed interval
There are two major parameters to design a drug dose regimen these are
• Dose size
• Dosing frequency i.e. intervals between two subsequent doses
Multiple dosage is used in case of being chronic disease two-compartments, heart diseases where long courses are required, unlike single dose regimen that is used in case of acute conditions.
Drug administration at fixed interval and dose
Dose:
Appropriate quantity of drug needed to produce required response.
It can be also defined as;
Quantitative dosage that estimates the quantity of the drug substance required to produce a biological response after administration is called dose size.
Fixed Dose
Repeated administration regimen is the most widely used approach to the expenditure of the therapy of drug.
• Upon the administration of the drug before the previously given dose is completely excreted from the body, results in the accumulation of the drug substance in the body.
• When the amount of the drug or the rate of drug into the body is equal to the rate of the drug out of the body means administration is equal to the elimination the average drug concentration reaches the plateau or steady state.
• At the plateau state, the quantity of the drug substance eliminated in each equal interval becomes equal to the quantity of the drug administered that can be determined by multiplying the dose with the bioavailability. Plasma concentration curve shows similar trend between the doses of drugs for each dosing interval.
Dose at Fixed Interval
Chronic diseases are treated usually by the multiple doses of drugs for example hypertension and arthritis. Upon the administration of the single dose of drug the drug plasma level quickly increases and reaches the MEC and then in the therapeutic window and after some time it falls below the MEC causing the reduction in the effect. Fan J, de Lannoy I., A. (2014 Jan 1)
If the drug is continuously administered at the fixed dose in regular intervals then the amount of drug in the body will start to rise until it will reach the steady state which means that constant plasma level and then peak concentration can be obtained by the preliminary dose.
Importance of Multiple Dose Regimen
Drug is accumulated inside the body if the second dose of the drug is administered to the person before the completion of the interval to eliminate the previously administered dose. Following the administration of single dose drug concentration rises and then falls below the minimum effective concentration MEC leading to the reduced therapeutic effect. Therefore, to enhance the therapeutic effect of the drug, it can be administered in multiple doses according to a regimen. Plasma concentration of the drug must remain within the therapeutic index to achieve the clinical outcome of the drug. Multiple drug dosage regimen is developed to provide the long-term delivery of the drug maintaining its concentration within narrow limits.
Criteria for Optimum Dosage Regimen
Optimum dosage regimen criteria depend upon these
• Drug concentration must be within narrow limits in the therapeutic index
• Should be convenient for the patient
Design of the drug dosage regimen is dependent upon these
• The dose size of the drug
• Dosage frequency i.e. time interval in between the doses
Superposition Principle
Superposition principle applies to the linear pharmacokinetic parameters, which means when the ADME is linear.
Hence the drug concentration after each dose can be added to calculate the concentration of drug after multiple doses.
Basic Assumptions for Superposition Principle
These are the two major basic assumptions for superposition principle
• Drug elimination must follow the linear kinetics
• PK of the drug does not change after multiple doses.
Drug Accumulation
Without the elimination of the already given dose, next administration of drug will contribute to the accumulation of drug in body by increasing the residual amount of drug that is already present in the body. Ambrose P. G., et al. (2007)
Smaller the interval of dosage administration as compared to the elimination interval then the amount of the drug that is residual will also be more upon the ingestion of the next dose as a result the drug can remain in body more extensively. Plateau state is ultimately achieved because the drug substance cannot accumulate over an indefinite period of time as a specific frequency of drug administration. The reason for this is that the elimination of a drug is actually dependent upon the concentration of the drug.
Higher the concentration of the drug more the elimination of drug from the body. If the two doses are missed, then the plateau concentration will fall below the therapeutic window as a result again a longer interval is required to regain that desired level of drug concentration in the plasma.
If the dose is divided in the several small doses, then the average plasma concentration of the drug shows small variation.
Time needed to reach the plateau state accumulation in the multiple dosage regimen is dependent upon the elimination rate. Plateau state declines to a recent value resulting in a new elimination rate. If there is no sufficient elimination of the drug, then the average plasma concentration of the drugs eliminated via renal pathway increases and can cause toxicity.
Intravenous Infusion
Intravenous therapy (IV) is a therapy that delivers fluids exactly into a vein. The intravenous route of administration can be used both for injections, using a syringe at higher pressures; as well as for infusions, typically using only the pressure supplied by gravity. Intravenous infusions are commonly pertained to as drips.
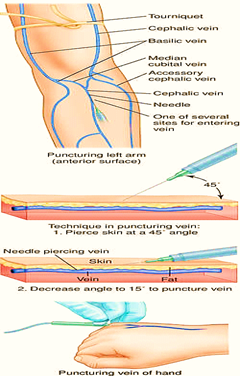
Figure 1
Method to administer an IV Infusion
Purpose of IV infusion
These are some of important purposes of IV infusion
• This strategy of liquid substitution is utilized most frequently
• Used to preserve liquid and electrolyte balance,
• Used to adjust liquid volume shortfalls after intemperate misfortune of body fluids,
• Used in patients incapable to require adequate volumes orally
• Maintenance of steady plasma concentration
• Dosage adjustment
Advantages of IV Infusion
These are the advantages of IV infusion
• Easy get to quick organization of solutions
• Continuous or irregular organization of nutrients
• Rapid changes in circulatory system
• Easy to screen conveyance of liquids, electrolytes, and supplements (for those with disabled GI tracts.)
One Compartment Model drugs IV Infusion
The one-compartment open model is the best method to portray the method of drug distribution and elimination within the body. This model assumes that the drug can enter or leave the body (i.e., the model is “open”), and the complete body acts like a single, uniform compartment.
Steady state or plateau Level
The plasma drug concentration–time curve of a drug given by consistent IV mixture is shown in Figure (graph).
Figure 2
Steady state level
Method
Upon infusing the drug via intravenous route at a constant rate will result in the first order kinetics in a frequent way to be attained. When the infusion is started then the amount of drug in the body is zero because no elimination is there. The addition of the drug concentration inside the body at the point when it is rising is increased but the elimination rate also. Therefore, the rate of elimination will continue to increase until it reaches the infusion rate. Drug concentration inside the blood will then remain constant and is said to be at the plateau state or the steady state concentration.
Infusion rate = Elimination rate
Rate of elimination of steady state = Cl × Cpss
Rate of administration = Cl × Cpss
Cl (total) = Cl (renal) + Cl (hepatic) + Cl (other)
Factors Affecting Steady state Plasma Concentration
Steady state plasma concentration is affected by the following factors
• Infusion rate which is directly proportional to the plateau state therefore increasing the rate in of the drug will increase the plasma concentration of drug at steady state.
• Plateau state is inversely proportional to the clearance rate of the drug.
The time to reach the plateau is determined by the elimination half-life of the drug, which results from clearance and volume of distribution. Thus, the Vd does not influence the steady state concentration but merely the time required to approach the plateau.
After 4 elimination half-lives the drug plasma concentration is 93,75% of the steady state plasma concentration. Likewise, when changing infusion rates, the time required to reach the new steady state also depends on the half-life of the drug.
When stopping an IV infusion, the decline in plasma drug concentration follows an exponential curve, as after an iv-bolus injection of the drug.
Clinical Outcomes
The infusion at a constant rate is utilized to guarantee a consistent introduction of the drug in the body over a large period of time.Giancotti, L. Talarico, V. Mazza, G., A. Marrazzo, S, Gangemi, P. Miniero, R. Bertini, M. (2019). To attain a therapeutic range concentration of the drug in the body a constant infusion rate of drug should be maintained.
Precautions
Intravenous infusions oughtto be ceased or infusion liquid replenished when the solution being administered is depleted.
Loading Dose
Loading dose is defined as the minimum effective dose which is given originally at a time to obtain the steady state plasma drug concentrations as early as possible.
Importance of Loading Dose
These are some of the important features of loading dose
• The main importance of loading dose is average plasma concentration at steady state as quickly as possible.
• In some cases, loading dose helps to urge therapeutic effect quickly.
• To attend fast plasma level.
• To attain fast action.
• To acquire desired concentration.
Calculations
We can calculate the loading
dose by the followings
Loading
dose= plasma concentration X volume of distribution / (Bioavailability X
fraction of drug salt)
These are the variables that
are used to calculate the loading dose:
Where Cp
is desired peak concentration of drug
Vd
is volume of distribution of drug in body
F is
bioavailability
S is fraction of drug salt form
which is active drug
For IV
administration the drug quickly and directly goes to the blood stream hence the
bioavailability of the drug will be equal to the 1. If the bioavailability of
the drug is to be decreased below the one then it will be due to the
Presystemic metabolism or limited absorption hence a greater loading dose will
be required.
Continuous
IV Infusion
We can give IV infusion of
various fluids and drugs using a specific infusion rate and modifying the
dosage regimen instead of giving a bolus at a time. This will avoid the
interruption
Table 1.
Complications with IV Infusion
|
Local Complications |
Systemic Complications |
|
· Infiltration |
· Embolism |
|
· Extravasation |
· Pulmonary
Embolism |
|
· Thrombosis |
· Air
Embolism |
|
· Thrombophlebitis |
· Catheter
Embolism |
|
· Phlebitis |
· Hematoma |
|
|
· Systemic
infection |
|
|
· Speed
shock |
|
|
· Circulatory
overload |
|
|
· Allergic
reaction |
IV
Infusion Devices
These are some of the IV diffusion
devices discussed
·
Cannula
·
IV tubing set and solution bag.
·
Tape
·
IV pole or pump.
Dripping
Rate
In an
IV (intravenous) treatment, the drip rate is interpreted as the rate of
application of a liquid drug required to deliver a certain dosage per minute.
An IV Infusion establish is used to allocate
fluids and medications directly into the blood stream. Infusion or flow rates
are modified to the desired drops per minute by a clamp on the tubing.
Calculation
of IV Drip Rates: IV Infusion Rates Formula
x Drop
Factor (gtts/mL) = IV infusion rat (gtts/min)
Note: Drops are always rounded to the nearest
whole number.
Complications
of IV Infusion
This is a table showing the
most common complications of IV infusion
Multiple
dose Regimen of Intravenous Administration
After a
single quick IV injection, the maximum quantity of drug in the body is equal to
the drug dose.
According
to first-order kinetics, the drug will be eliminated for a one-compartment open
model. Gai, X. Shen, N. He,
B. Zhou, Q. Bo, S. Li, X. Zhai, S. Yin, A. Lu, W. (2015).
If ? is
identical to the dosage interval, it is possible to determine the amount of
drug that remains in the body after several hours.
Elimination
constant and dosing interval determines the amount of the drug remaining in the
body. as follows:
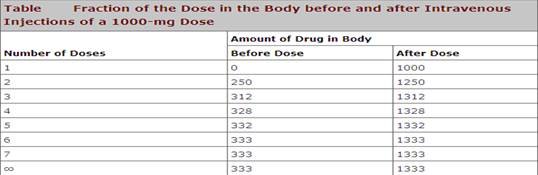
F=db/do
Amount
of drug in the body will be less if the dosing interval will be less.
If the
dose of 100 mg is given after each half-life then the dosing interval will be
equal to half life.
After
the first half-life of drug it will be 50mg.
After
the second half-life of drug it will be 150mg.
After
the third half-life of drug it will be 75mg.
After
the fourth half-life of drug it will be 175mg.
This
will continue until an equilibrium will be attained
Dmax =200mg and Dmin
=100mg
Therefore
the subsequent administration of the drug increases the body load and there is
an absolute quantity of drug substance that is eliminated per unit time that’s
what is responsible for the first order kinetics means that drug going into the
body is equal to the drug going out of the body.
Table 2: Fraction of the dose in the body before and after Intravenous administration
Single Dose Regimen of IV Administration
When the interval of therapy is minor than the therapeutic activity of drug simple dose are provided in this manner. (Bastone, E. B., Li, S. C., Ioannides-Demos, L. L., Spicer, W. J., McLean, A. J. 1993) These are used in cases where therapeutic activity is required within no time or within seconds.
Conclusion
This review illustrates the importance of multiple dosage regimen that is used in treatment of chronic diseases as heart diseases and arthritis etc. Maintenance dosage regimen helps to improve clinical efficacy and therapeutic activity of the drugs decreasing the side effects and adverse drug reactions.
References
- Agashe, H., Sahoo, K., Lagisetty, P., & Awasthi, V. (2011). Cyclodextrin-mediated entrapment of curcuminoid 4-[3, 5-bis (2-chlorobenzylidene- 4-oxo-piperidine-1-yl)-4-oxo-2-butenoic acid] or CLEFMA in liposomes for treatment of xenograft lung tumor in rats. Colloids and Surfaces B: Biointerfaces, 84(2), 329-337.
- Allen, T. M. (2002). Ligand-targeted therapeutics in anticancer therapy. Nature Reviews Cancer, 2(10), 750.
- Anselmo, A. C., & Mitragotri, S. (2014). Cell- mediated delivery of nanoparticles: taking advantage of circulatory cells to target nanoparticles. Journal of controlled release, 190, 531-541.
- Attama, A., Reichl, S., & Müller-Goymann, C. (2009). Sustained release and permeation of timolol from surface-modified solid lipid nanoparticles through bioengineered human cornea. Current eye research, 34(8), 698-705.
- Bhagwat, R., & Vaidhya, I. (2013). Novel drug delivery systems: An overview. International Journal of Pharmaceutical Sciences and Research, 4(3), 970.
- Booysen, L., Kalombo, L., Brooks, E., Hansen, R., Gilliland, J., Gruppo, V., . . . Kotze, A. (2013). In vivo/in vitro pharmacokinetic and pharmacodynamic study of spray-dried poly- (dl-lactic-co-glycolic) acid nanoparticles encapsulating rifampicin and isoniazid. International journal of pharmaceutics, 444(1- 2), 10-17.
- Dalpiaz, A., Vighi, E., Pavan, B., & Leo, E. (2009). Fabrication via a nonaqueous nanoprecipitation method, characterization and in vitro biological behavior of N6-cyclopentyladenosine-loaded nanoparticles. Journal of pharmaceutical sciences, 98(11), 4272-4284.
- Danhier, F., Ansorena, E., Silva, J. M., Coco, R., Le Breton, A., & Préat, V. (2012). PLGA-based nanoparticles: an overview of biomedical applications. Journal of controlled release, 161(2), 505-522.
- Douglas, K. L., & Tabrizian, M. (2005). Effect of experimental parameters on the formation of alginate-chitosan nanoparticles and evaluation of their potential application as DNA carrier. Journal of Biomaterials Science, Polymer Edition, 16(1), 43-56.
- El-Nour, K. M. A., Eftaiha, A. a., Al-Warthan, A., & Ammar, R. A. (2010). Synthesis and applications of silver nanoparticles. Arabian journal of chemistry, 3(3), 135-140.
- Emeje, M. O., Obidike, I. C., Akpabio, E. I., & Ofoefule, S. I. (2012). Nanotechnology in drug delivery. Recent advances in novel drug carrier systems, 69-106.
- Galindo-Rodriguez, S., Allemann, E., Fessi, H., & Doelker, E. (2004). Physicochemical parameters associated with nanoparticle formation in the salting-out, emulsification- diffusion, and nanoprecipitation methods. Pharmaceutical research, 21(8), 1428-1439.
- Ganachaud, F., & Katz, J. L. (2005). Nanoparticles and nanocapsules created using the Ouzo effect: spontaneous emulsification as an alternative to ultrasonic and high-shear devices. ChemPhysChem, 6(2), 209-216.
- Gao, Y., Chen, L., Zhang, Z., Chen, Y., & Li, Y. (2011). Reversal of multidrug resistance by reduction-sensitive linear cationic click polymer/iMDR1-pDNA complex nanoparticles. Biomaterials, 32(6), 1738-1747.
- Geçer, A., Yıldız, N., Çalımlı, A., & Turan, B. (2010). Trimethyl chitosan nanoparticles enhances dissolution of the poorly water soluble drug Candesartan-Cilexetil. Macromolecular research, 18(10), 986-991.
- Greish, K. (2010). Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting Cancer Nanotechnology (pp. 25-37): Springer
- Hans, M. L., & Lowman, A. M. (2002). Biodegradable nanoparticles for drug delivery and targeting. Current Opinion in Solid State and Materials Science, 6(4), 319-327.
- In, G. K., & Nieva, J. (2015). Emerging chemotherapy agents in lung cancer: nanoparticles therapeutics for non-small cell lung cancer. Translational Cancer Research, 4(4), 340-355.
- skandar, F., Gradon, L., & Okuyama, K. (2003). Control of the morphology of nanostructured particles prepared by the spray drying of a nanoparticle sol. Journal of Colloid and Interface Science, 265(2), 296-303.
- Jain, S., Mittal, A., & K Jain, A. (2011). Enhanced topical delivery of cyclosporin-A using PLGA nanoparticles as carrier. Current nanoscience, 7(4), 524-530.
- Jain, S., Mittal, A., K Jain, A., R Mahajan, R., & Singh, D. (2010). Cyclosporin A loaded PLGA nanoparticle: preparation, optimization, in-vitro characterization and stability studies. Current Nanoscience, 6(4), 422-431.
- Khan, I., Saeed, K., & Khan, I. (2017). Nanoparticles: Properties, applications and toxicities. Arabian journal of chemistry.
- Kim, B., Hwang, S., Park, J., & Park, H. J. (2002). Preparation and characterization of drug- loaded polymethacrylate microspheres by an emulsion solvent evaporation method. Journal of microencapsulation, 19(6), 811-822.
- Kostag, M., Köhler, S., Liebert, T., & Heinze, T. (2010). Pure cellulose nanoparticles from trimethylsilyl cellulose. Paper presented at the Macromolecular symposia.
- Kozek, K. A., Kozek, K. M., Wu, W.-C., Mishra, S. R., & Tracy, J. B. (2013). Large-scale synthesis of gold nanorods through continuous secondary growth. Chemistry of Materials, 25(22), 4537- 4544.
- Kreyling, W. G., Semmler, M., & Möller, W. (2004). Dosimetry and toxicology of ultrafine particles. Journal of Aerosol Medicine, 17(2), 140-152
- Kumar, P., Sulochana, P., Nirmala, G., Haridattatreya, M., & Satchidanandam, V. (2004). Conserved amino acids 193-324 of non-structural protein 3 are a dominant source of peptide determinants for CD4 and CD8 T cells in a healthy Japanese encephalitis virus- endemic cohort. Journal of General Virology, 85(5), 1131-1143.
- Lamprecht, B., Schider, G., Lechner, R., Ditlbacher, H., Krenn, J. R., Leitner, A., & Aussenegg, F. R. (2000). Metal nanoparticle gratings: influence of dipolar particle interaction on the plasmon resonance. Physical review letters, 84(20), 4721.
- Laurent, S., Forge, D., Port, M., Roch, A., Robic, C., Vander Elst, L., & Muller, R. N. (2008). Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chemical reviews, 108(6), 2064-2110.
- Liu, P. (2013). Modification strategies for carbon nanotubes as a drug delivery system. Industrial & Engineering Chemistry Research, 52(38), 13517-13527.
- Liu, Z., Wu, Y., Guo, Z., Liu, Y., Shen, Y., Zhou, P., & Lu, X. (2014). Effects of internalized gold nanoparticles with respect to cytotoxicity and invasion activity in lung cancer cells. PLoS One, 9(6), e99175.
- Lu, Z., Bei, J., & Wang, S. (1999). A method for the preparation of polymeric nanocapsules without stabilizer. Journal of Controlled Release, 61(1-2), 107-112.
- Marelli, U. K., Rechenmacher, F., Sobahi, T. R. A., Mas-Moruno, C., & Kessler, H. (2013). Tumor targeting via integrin ligands. Frontiers in oncology, 3, 222.
- Mishra, B., Patel, B. B., & Tiwari, S. (2010). Colloidal nanocarriers: a review on formulation technology, types and applications toward targeted drug delivery. Nanomedicine: Nanotechnology, biology and medicine, 6(1), 9- 24.
- Mohanraj, V., & Chen, Y. (2006). Nanoparticles-a review. Tropical journal of pharmaceutical research, 5(1), 561-573.
- Morones, J. R., Elechiguerra, J. L., Camacho, A., Holt, K., Kouri, J. B., RamÃÂrez, J. T., & Yacaman, M. J. (2005). The bactericidal effect of silver nanoparticles. Nanotechnology, 16(10), 2346.
- Mu, L., & Feng, S. (2003). A novel controlled release formulation for the anticancer drug paclitaxel (Taxol®): PLGA nanoparticles containing vitamin E TPGS. Journal of controlled release, 86(1), 33-48.
- Muhamad12, I. I., Selvakumaran, S., & Lazim, N. A. M. (2014). Designing polymeric nanoparticles for targeted drug delivery system. Nanomed, 287, 287.
- Nel, A., Xia, T., Mädler, L., & Li, N. (2006). Toxic potential of materials at the nanolevel. science, 311(5761), 622-627.
- Nicoli, S., Santi, P., Couvreur, P., Couarraze, G., Colombo, P., & Fattal, E. (2001). Design of triptorelin loaded nanospheres for transdermal iontophoretic administration. International journal of pharmaceutics, 214(1-2), 31-35
- Ochekpe, N. A., Olorunfemi, P. O., & Ngwuluka, N. C. (2009). Nanotechnology and drug delivery part 2: nanostructures for drug delivery. Tropical journal of pharmaceutical research, 8(3).
- Ouyang, Y., Shi, H., Fu, R., & Wu, D. (2013). Highly monodisperse microporous polymeric and carbonaceous nanospheres with multifunctional properties. Scientific reports, 3, 1430.
- Pandey, N., Dhiman, S., Srivastava, T., & Majumder, S. (2016). Transition metal oxide nanoparticles are effective in inhibiting lung cancer cell survival in the hypoxic tumor microenvironment. Chemico-biological interactions, 254, 221-230.
- Poland, C. A., Duffin, R., Kinloch, I., Maynard, A., Wallace, W. A., Seaton, A., . . . Donaldson, K. (2008). Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nature nanotechnology, 3(7), 423.
- Rangari, A. T., & Ravikumar, P. (2015). Polymeric nanoparticles based topical drug delivery: an overview. Asian Journal of Biomedical and Pharmaceutical Sciences, 5(47), 5.
- Rodrigues, S., da Costa, A. M. R., & Grenha, A. (2012). Chitosan/carrageenan nanoparticles: Effect of cross-linking with tripolyphosphate and charge ratios. Carbohydrate polymers, 89(1), 282-289.
- Soppimath, K. S., Aminabhavi, T. M., Kulkarni, A. R., & Rudzinski, W. E. (2001). Biodegradable polymeric nanoparticles as drug delivery devices. Journal of controlled release, 70(1-2), 1-20.
- Stevanovic, M., & Uskokovic, D. (2009). Poly (lactide-co-glycolide)-based micro and nanoparticles for the controlled drug delivery of vitamins. Current nanoscience, 5(1), 1-14
- Venkatesh, D. N., Baskaran, M., Karri, V. V. S. R., Mannemala, S. S., Radhakrishna, K., & Goti, S. (2015). Fabrication and in vivo evaluation of Nelfinavir loaded PLGA nanoparticles for enhancing oral bioavailability and therapeutic effect. Saudi Pharmaceutical Journal, 23(6), 667-674.
- Wang, Y., Wei, X., Zhang, C., Zhang, F., & Liang, W. (2010). Nanoparticle delivery strategies to target doxorubicin to tumor cells and reduce side effects. Therapeutic delivery, 1(2), 273- 287.
- Xu, Y., Roy, R., Cassaro, G., & Ramsden, J. (2009). Development of a cost estimating framework for nanotechnology based products Global Perspective for Competitive Enterprise, Economy and Ecology (pp. 193-201): Springer.
- Yang, W.-W., & Pierstorff, E. (2012). Reservoir- based polymer drug delivery systems. Journal of laboratory automation, 17(1), 50-58.
Cite this article
-
APA : Ayub, A., Haq, A. u., & Rehman, M. (2019). A Comprehensive Insights of the Multiple Dose Regimen. Global Drug Design & Development Review, IV(I), 34-40. https://doi.org/10.31703/gdddr.2019(IV-I).04
-
CHICAGO : Ayub, Ayesha, Abid ul Haq, and Mubashar Rehman. 2019. "A Comprehensive Insights of the Multiple Dose Regimen." Global Drug Design & Development Review, IV (I): 34-40 doi: 10.31703/gdddr.2019(IV-I).04
-
HARVARD : AYUB, A., HAQ, A. U. & REHMAN, M. 2019. A Comprehensive Insights of the Multiple Dose Regimen. Global Drug Design & Development Review, IV, 34-40.
-
MHRA : Ayub, Ayesha, Abid ul Haq, and Mubashar Rehman. 2019. "A Comprehensive Insights of the Multiple Dose Regimen." Global Drug Design & Development Review, IV: 34-40
-
MLA : Ayub, Ayesha, Abid ul Haq, and Mubashar Rehman. "A Comprehensive Insights of the Multiple Dose Regimen." Global Drug Design & Development Review, IV.I (2019): 34-40 Print.
-
OXFORD : Ayub, Ayesha, Haq, Abid ul, and Rehman, Mubashar (2019), "A Comprehensive Insights of the Multiple Dose Regimen", Global Drug Design & Development Review, IV (I), 34-40
-
TURABIAN : Ayub, Ayesha, Abid ul Haq, and Mubashar Rehman. "A Comprehensive Insights of the Multiple Dose Regimen." Global Drug Design & Development Review IV, no. I (2019): 34-40. https://doi.org/10.31703/gdddr.2019(IV-I).04