01 Pages : 1-8
Abstract
A process in which drug comes out from drug product and exposed to ADME and finally become accessible for pharmacological activity is known as drug release and mechanism involves the study of its rate and factors influencing its rate. Factors influencing the rate of drug release are drug related, polymer related and formulation variables. Drug delivery systems are discriminated on the basis of way drug is delivered, which are immediate release and modified release. Parenteral dosage forms are the most prominent among different dosage forms. The mechanisms involved in drug release are diffusion and dissolution-controlled processes. Nasal disposal basically depends on the particle size, its geometry and rate of airflow. Nasal sprays are commonly used than powders and gels. Buccal and sublingual systems show more effective drug release mechanisms than oral and transdermal systems. Main focus of paper is to elaborate different mechanisms performed by drug delivery systems for drug release.
Key Words
Drug Release Mechanisms, Dosage Forms, Drugs, Parenteral, Diffusion
Introduction
Drug Release
A process via which a drug is left from a drug product and is exposed to ADME and ultimately becomes accessible for pharmacological action is called drug release.
Drug Release Mechanisms
Drug Release Mechanisms is the amount of drug release from a particular dosage form after dissolution and the study of aspects affecting release rate of the drug. Dissolution and release of drugs are essential terms for solid and semi-solid dosage forms which are involved in delivering the drugs over the intended period of time.
Terms Involved in Drug Release Mechanism
They include diffusion, dissolution, swelling, precipitation and/or degradation.
Dissolution
it involves the transfer of mass from a solid to liquid phase.it consists of 2 consecutive phases.
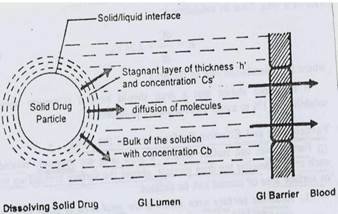
Noyes–Whitney Equation
Noyes-Whitney-Nernst equation explains drug dissolution process of solid dosages. (Hattori, Haruna& Otsuka 2013)
Rate of dissolution= Ks (Cs-C)
Figure 1
Mathematical Model for Drug Dissolution Noyes- Whitney Equation
Diffusion
It is the wetting of surface and medium ingression into tablet, drug’s dissolution, and diffusion of the soluble drug across hydrated matrix into the medium.
Factors that affect Drug Release’s Mechanism
Several factors influenced the drug release’s mechanism.
Drug Related Factors
Drug Solubility
Faster release is shown by highly soluble drugs, while incomplete release is often shown by poorly water-soluble drugs (<0.01 mg/mL) often show incomplete release.
Dosage
The rate of release s increased by increased drug content at persistent polymer content and hence, higher chemical gradient at the diffusion front.
MW and Size
When matrix is completely hydrated, the drug’s diffusion coefficient slowly changes from near zero to maximum. The diffusion coefficient is dependent on MW, solute molecule’s diameter, and diffusion medium’s viscosity. Due to constrain imposed by the aqueous gel structure, drugs with a MW of >500Da are poorly diffusible in hydrophilic matrices.
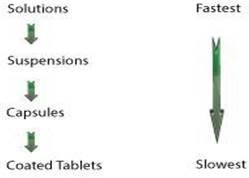
Particle Size and Shap
In terms of effective surface area, drug release is determined by particle size and shape of soluble drugs.
Figure 2
Dissolution of Various Dosage forms
Polymer Related Factors
Release happens by two mechanisms, drug will either diffuse through polymer or polymer erode. Diffusion of drug is increased as polymer content increases and the interaction between the polymer and solute. The properties of polymers like viscosity, etc must be measured. Viscosity of gel increases with an increase in polymer content, leading to decrease in effective diffusion coefficient of the drug and rate of drug release falls.
Formulation Variables
Increased surface area is provided by an increased tablet size which leads to total equilibrium in the release rate. drug release may be increased by adding surfactant through improved wetting. Formulation components like binding agents can hinder drug release, plasticizers may increase drug-release rates and lubricants will delay drug release.
Differentiating drug delivery systems according to their composition:
Dissolution Controlled System
The rate controlling step is dissolution in this system. The drug is implanted in erodible matrix in this system.
It has 2 kinds:
a) Encapsulation dissolution-controlled system
b) Matrix dissolution-controlled system
Diffusion Controlled System
The rate limiting step is the diffusion of drug through membrane barrier that is water insoluble. This system has 2 kinds:
a) Reservoir diffusion system
b) Matrix diffusion system
Dissolution and Diffusion-Controlled System
Moderately soluble membrane encloses a drug core. dissolution of the parts of membrane creates pores that allow entrance of aqueous medium into core, therefore dissolution of drug occurs and permit diffusion of dissolved drugs out of the system. (Simon, Bolisetty&Erazo, 2011).
Water Penetration-Controlled System
Rate controlling step is the penetration of water into the system is the rate controlling step.
It has 2 kinds:
a) Swelling controlled system
b) Osmotically controlled system
Figure 3
Reservoir diffusion Swelling and b-matrix diffusion swelling
Swelling Controlled System
They are primarily dry and absorb water and other fluid when sited in body and swells.
Osmotically Controlled System
They are devised by enclosing an osmotic drug core which contains an osmotically active drug or combination of osmotically inactive drug with a salt (NaCl). A slope of osmotic pressure has been formed, where the drug solutes have been continually driven out over a prolonged period of time through delivery orifice. (Gupta et al., 2010)
Chemically Controlled System
They alter their chemical structure when visible to the biological fluid. The polymer is degraded into biologically safe and smaller moieties as a result of hydrolysis.
It has 2 kinds:
a) Erodible system
b) Pendent chain system
Erodible System
This system involves the occurrence of mechanism of drug release by erosion. Erosion may have 2 kinds:
a) Bulk erosion
b) Surface erosion
Pendent Chain System
It consists of linear or homo or copolymers attached to drug. The drug is release from polymer by hydrolysis or enzymatic degradation of the linkages.
Hydrogels
Hydrogels are water swollen 3-D structure made up of chiefly hydrophilic polymers. They are rendered insoluble because of chemical and physical cross links.
Classification of Delivery Systems of Drugs Based on Drug Release Mechanisms
Drug delivery systems are differentiated on the basis of drug release. Broadly, they are classified as:
Immediate Release
after administration there is immediate release of drug.
Modified Release
after administration of drug its released time is delayed. Modified release systems are classed as:
Delayed Release
After initial administration release of drug occurs just at some spot.
Extended Release
to reduce dosing frequency, it prolongs release
Immediate Release
Some dosage forms are released immediately or instantly after administration into body. It is the required for some for some therapeutic causes. Example includes IV injections and infusions which have fast onset of action and after administration show pharmacological outcome in few seconds.
Delayed Release System
Delayed release dosage form is the formulation of such systems that release the active ingredients after some time of administration instead of immediately. These systems are very beneficial for those drugs which undergo degradation in the acidic environment of stomach. So, in such circumstances there is delay in the release of drug until it reaches the small intestine. For this purpose, polymers are often used. The dosage form either tablet or capsule before tableting undergoes coating of a polymer. When such dosage form moves from acidic to basic pH environment i.e., from stomach to small intestine the polymer dissolves so a polymer dissolves according to pH and the drug releases. Once this process occurs the drug releases immediately and the curve obtained between plasma concentration and time is exactly the same as for immediate dosage forms.
Extended Release
The purpose of using the extended-release dosage forms is to extend the time period for the release of drug. In this way there is decrease in the dosing frequency of drug. Sustained – or controlled release dosage forms play important role in achieving extended-release forms.
Sustained Release
These systems maintain rate of drug release over prolonged period. This can be achieved by use of polymers which involve in the coating of tablets or granules or for formation of matrix system in which there is usually dispersion of drug occurs.
Controlled Release Systems
Polymeric matrix which is biodegradable is usually used for this system. Matrix consists of therapeutic agent in the dispersed or enclosed form. So, this leads to the formation of complex heterogeneous release pattern. First there is immediate release of drug that means it’s not protected well by carrier. And then from polymeric matrix there is controlled release of drug occurs. Mechanisms involved in this system are combinations of diffusion and degradation.
Optimum Release Profile
It is in the favor of some diseases having varied drug release rate depending on requirements of patient or their body’s circadian rhythms. It can be explained through an example regarding the concentration of insulin that is required in greater amount after meal and B.P also varies as it is higher in morning and afternoon while falls during night. This leads to analysis into thus known as feedback regulated drug delivery system in which with help of sensor the concentration of drug can be measured, and drug release is either enhanced or decreased depending on the ideal drug concentration.
Targeted Release Dosage Forms
The purpose of drug targeting is to manage disposition of drug within a body in a way that majority of dose particularly binds to target tissue at cellular or subcellular level. The purpose of this is to increase activity and selectivity of drug and to decrease its side effects. Such systems are designed in which targeting is done passively by altering natural conditions of target tissues to achieve drug targeting. On the other hand, drug targeting can be achieved directly by targeting groups so that they bind on cells at specific receptors.
Drug Release Mechanisms from Different Dosage Forms
Delivery of Drug Via Parenteral Route
Parenteral dosage forms are the most prominent among different dosage forms and then aerosols and nasal dosage forms stand. The mechanisms involved in drug release are diffusion and dissolution-controlled processes. Mechanisms of drug release mainly depends on the excipients, types of excipients, their amount, manufacturing methods, dosage form’s geometry, routes of administration, drug’s pharmacokinetic and physico-dynamics.
Delivery of drug via respiratory route
Depositing of drugs respiratory tract is brought about by following mechanisms:
Depositing Via Impaction Due to Inertia
This deposition is brought about by inertia of aerosol particles. Particles possessing high mass or high velocity tend to show greater landing distances and have greater probability of sticking on the interior periphery of respiratory pathway. (Newman S. P., 2016)
Depositing Via Sedimentation
Here, tiny particles (0.5 to 3 ?m) lands via sedimentation to bronchioles and alveoli.
Depositing Via Diffusion
This mechanism does not depend upon the density of a particle but increases when we decrease the size of the particle and depends on time for which particle resides and this deposition can me more significant if breath is held in for some time.
Depositing Via Interference
Generally, exists for particles having unequal breadths and widths.
Depositing Via Electrostatic Effect
It exists for particles possessing some charge. Increased landing of aerosol particles in lung lining is brought about with greater percentage of particles of size less than 5 ?m. Factors responsible for adhesion of particles as well as their dispersion include PK characteristics of carrier and drug particle, for instance ratio between drug and its carrier. Drug deposition of drug is mostly seen with carriers of small size and presence of particles in greater percentage.
Delivery of Drug Via Nasal Route
It is profoundly dependent upon size of particle or droplet, rate of air flow, and topology of nasal passage. Particles inhaled land majorly via impaction due to inertia. Relocation via clearance through mucus and ciliary brings a further subsidiary landing of drug.
The bioavailability as well as absorption via nasal passage is influenced by PK characteristics of drug. Nasal
delivery via suspension dosage forms is not in normal practice owing to finite volume of water present in nasal chamber for dissolution.
In case of dry powders or powders for suspension for nasal delivery, rate of dissolution influence rate of absorption. Particles that are to land in nasal space normally be in dissolved form before their absorption. Bioavailability definitely reduces when drug particles leave the tract before they are absorbed.
Delivery of Drug Via Buccal and Sublingual Route
Movement of drug to traverse mucosa oral passageway is brought about by para-cellular and trans-cellular course.
Absorption predominantly takes place via passive diffusion. Absorption of drug is influenced by its lipophilicity, molecular weight as well as dissolubility at the main absorption site. Greater potency is a key for pronounced drug delivery via buccal and sublingual course, owing to the confined surface area for drug absorption. (Senel et al., 2012)
Delivery of Drug Via Oral Route
Drug absorption rate of oral dosage form is governed by the PK characteristics of drug, features related to the dosage form formulation, and state of body with respect to body’s physiology. PK characteristics of drug influencing the absorption constitute (a)dissolution rate, (b)extent to which the drug ionizes, and (c)molecular weight. Drugs that do not ionize possess higher potential to permeate through the membrane. Majority of the drugs get absorbed in colon from its small intestine portion, despite the extent of ionization. Nevertheless, tiny particles accretion sometimes may tend to expand the essential surface area, bringing about constant or decreasing rate of dissolution and hence the resulting bioavailability.
Delivery of Drugs Via Topical and Transdermal Course
For transdermal delivery, the drugs must infiltrate skin barrier in quantities that are enough to bring about systemic effect without being influenced by enzymes present in the uppermost layer of skin i.e. epidermis. Potency to bring about therapeutic effect must be achieved in doses lesser than 10mg.
Two fundamental constitutional layout of transdermal patch system govern the release of drug from the system:
Matrix System also Known as Monolithic System
In this type of system, the drug binds to polymer considered for making matrix should be inert in nature and should bind the drug and regulate the liberation of drug from patch.
Depot System
it is also called depot system. The matrix is made up of polymer, but it does not regulate the outflow of drug. Here is a membrane that exists between drug matrix and an adhesive layer. This membrane serves to be rate limiting owing to be a bottleneck step for the release of drug from the patch.
Conclusion
It is finally concluded that the need for different release mechanism is significant to cater the needs different patients, different diseases, different drugs, preference in a specific case for the maximum benefit of patient and to provide the patient with a drug which when given has such release mechanism that its benefits outweigh its risks as far as the case of a patient is concerned and is beneficial for improving the life of a patient. For the purpose stated above, one should also consider the point that the treatment focused on a certain release mechanism must not be financially unbearable to the patient. immediate release drugs are given when we have to rehabilitate the patient as soon as possible or when if the patient is not given immediate treatment, his or her case would become detrimental e.g., angina pectoris, coma, anaphylaxis shock etc. Among modified release dosage forms, delayed release dosage forms are given when the drug needs to bypass certain length of its overall course so that it can just work on its desired site of action and would not be degraded before it reaches its actual site. Extended-release dosage forms are a choice when there is a need for reduced frequency of dosing. Extended-release dosage forms are employed when it calls for a drug to be released for a prolonged duration of time. The main benefit of this release is reduced frequency. Sustained release mechanism is employed to maintain a fixed concentration over a particular period of time with least side effects. Controlled release dosage forms are suitable when it calls for the therapeutic substances to be released for longer duration of time with controlled rates and mostly for days to weeks and even months and many a times year also. Its main benefit is minimal blood plasma drug levels and low toxicity.
IV injection is an immediate release dosage form with bioavailability of 100 percent which seems an ideal case but exists in reality. IM preparations are given in case of drugs when they are poorly hydrophilic and in situations where systemic as well as local or anyone of these side effects occur due to increased peak drug concentrations.
Drug delivery by respiratory includes mechanisms such as impaction, sedimentation, diffusion, interference, electrostatic effect. The nasal route follows mainly the mechanism of impaction due to inertia. In buccal and sublingual routes, absorption occurs significantly via passive diffusion and the bottleneck step there is confined volume of fluid for effective absorption.
Drug delivery via transdermal route includes absorption via the use of adhesive aka sticky devices that regulate the release and absorption of a particular amount of drug over a period of time and the basic infrastructure of these devices is different to serve the purpose different on case-to-case basis. Some devices are disposable while others are re-usable owing to their sterilization and economical affective conditions and their frequent and easy availability.
References
- Balant L. P. (1993). Regulatory aspects of modified release dosage forms: clinical studies. Bollettinochimicofarmaceutico, 132(5), 143- 149.
- Garbacz, G., & Klein, S. (2012). Dissolution testing of oral modified-release dosage forms. TheJournal of pharmacy and pharmacology, 64(7), 944- 968.
- Gupta, B. P., Thakur, N., Jain, N. P., Banweer, J., & Jain, S. (2010). Osmotically controlled drug delivery system with associated drugs. Journal of pharmacy & pharmaceutical sciences: a publication of the Canadian Society for Pharmaceutical Sciences, Societecanadienne des sciences pharmaceutiques, 13(4), 571-588.
- Hattori, Y., Haruna, Y., & Otsuka, M. (2013). Dissolution process analysis using model-free Noyes-Whitney integral equation. Colloids and surfaces. B, Biointerfaces, 102, 227-231.
- Keraliya, R. A., Patel, C., Patel, P., Keraliya, V., Soni, T. G., Patel, R. C., & Patel, M. M. (2012). Osmotic drug delivery system as a part of modified release dosage form. ISRN pharmaceutics, 2012, 528079.
- Mehuys, E., &Vervaet, C. (2010). Systèmesd'administration par voieorale àlibérationcontrôlée [Oral controlled release dosage forms]. Journal de pharmacie de Belgique, (2), 34-38.
- Newman S. P. (2016). Drug delivery to the lungs: challenges and opportunities. Therapeutic delivery, 8(8), 647-661.
- Şenel, S., Rathbone, M. J., Cansız, M., &Pather, I. (2012). Recent developments in buccal and sublingual delivery systems. Expert opinion on drug delivery, 9(6), 615-628.
- Siepmann, J., &Siepmann, F. (2012). Modeling of diffusion-controlled drug delivery. Journal of controlled release: official journal of the Controlled Release Society, 161(2), 351-362.
- Simon, L., Bolisetty, P., &Erazo, M. N. (2011). Dynamics of dissolution and diffusion- controlled drug release systems. Current drug delivery, 8(2), 144-151.
- Singh Malik, D., Mital, N., & Kaur, G. (2016). Topical drug delivery systems: a patent review. Expert opinion on therapeutic patents, 26(2), 213-228.
- Verma, R. K., Mishra, B., & Garg, S. (2000). Osmotically controlled oral drug delivery. Drug development and industrial pharmacy, 26(7), 695-708.
- Vilar, G., Tulla-Puche, J., &Albericio, F. (2012). Polymers and drug delivery systems. Current drug delivery, 9(4), 367-394.
Cite this article
-
APA : Shahid, H., Ahmed, A., & Ashraf, A. (2016). An Insight About the Drug Release Mechanisms from Different Dosage Forms. Global Drug Design & Development Review, I(I), 1-8. https://doi.org/10.31703/gdddr.2016(I-I).01
-
CHICAGO : Shahid, Hafsa, Ayesha Ahmed, and Ammarah Ashraf. 2016. "An Insight About the Drug Release Mechanisms from Different Dosage Forms." Global Drug Design & Development Review, I (I): 1-8 doi: 10.31703/gdddr.2016(I-I).01
-
HARVARD : SHAHID, H., AHMED, A. & ASHRAF, A. 2016. An Insight About the Drug Release Mechanisms from Different Dosage Forms. Global Drug Design & Development Review, I, 1-8.
-
MHRA : Shahid, Hafsa, Ayesha Ahmed, and Ammarah Ashraf. 2016. "An Insight About the Drug Release Mechanisms from Different Dosage Forms." Global Drug Design & Development Review, I: 1-8
-
MLA : Shahid, Hafsa, Ayesha Ahmed, and Ammarah Ashraf. "An Insight About the Drug Release Mechanisms from Different Dosage Forms." Global Drug Design & Development Review, I.I (2016): 1-8 Print.
-
OXFORD : Shahid, Hafsa, Ahmed, Ayesha, and Ashraf, Ammarah (2016), "An Insight About the Drug Release Mechanisms from Different Dosage Forms", Global Drug Design & Development Review, I (I), 1-8
-
TURABIAN : Shahid, Hafsa, Ayesha Ahmed, and Ammarah Ashraf. "An Insight About the Drug Release Mechanisms from Different Dosage Forms." Global Drug Design & Development Review I, no. I (2016): 1-8. https://doi.org/10.31703/gdddr.2016(I-I).01