Abstract
Pharmacokinetics can be defined as what the body does to a drug. The basic parameters of pharmacokinetics are discussed here including absorption, distribution, metabolism, and excretion. Characteristics and pathways taken by these drugs are determined by these parameters. The mechanism followed by these parameters are also discussed. Furthermore, the factors affecting these parameters including physicochemical factors, physical factors and pharmaceutical factors are also explored. Different routes of drug absorption and main barriers to drug distribution are also explained. The pharmacokinetic values namely acid dissociation constant, bioavailability and solubility are briefly explained. There is a detailed insight into the pathways of metabolism (Phase I and II reactions) and excretion.
Key Words
Pharmacokinetics, Absorption, Distribution, Metabolism
Introduction
Pharmacokinetics
can be described as the movement of the drug substance through, into and out of
the body. ADME characteristics along with rate and extent are also involved in
pharmacokinetics. The pharmacokinetics of a drug depends on many factors that includes
its apparent Vd, physicochemical properties, intrinsic clearance, and its
interaction with different types of tissues. It serves as a useful tool for not
only determining the safety and efficacy of the drug but also for describing
the comparison of disposition of formulations and thus can be employed for
tailoring compound to a new dosage regimen.
The pharmacokinetic principles can be applied
to various biomedical fields like Dosage form evaluation, toxicological
studies, Drug formulation evaluation, evaluation of organ function & dosing
regimen design etc. Information pharmacokinetic characters of drugs and factors
affecting them for designing an effective drug delivery system is very useful.
Basic Parameters of Pharmacokinetics
Ø Absorption
Ø Distribution
Ø Metabolism
Ø Excretion
The onset, duration, and intensity of a drug's
effect can be determined by drug pharmacokinetics. Intensity of effect and
concentration of the drug are interrelated at the site of action, which depends
on its pharmacokinetic properties
Clinical
Pharmacokinetics
Application of the
principles of pharmacokinetics in the efficient and safe management of the
therapeutics drugs
individualized to a patient. The main goal of
clinical pharmacokinetics is to decrease the toxicity and enhance the efficacy
of the drug.
Absorption
Movement of the drug
substance from the site where it is administered to the systemic circulation.
The effectiveness of drug can only be assessed by its concentration at site of
action. The extent as well as the rate of absorption depend on different
parameters.
Mechanism
of Drug Absorption
There are 6 major
mechanism of drug transport
Transcellular
Route
It is the pathway
most common for hydrophobic molecules. Hydrophobic properties of such
substances allow them to pass through the cell membranes. This process requires
energy.
Example
calcium binding
proteins transport the calcium ions across the cell membrane by this pathway.
Paracellular
Route
It is a pathway in
which cells in the epithelium adhere to each other in the monolayers by tight
junctions and cell pass through the intercellular spaces between them. It is
preferred for hydrophilic molecules and small molecules without expenditure of
energy These have specific junctional complexes: Zona occludens, Zona adherens,
Desmosomes & Gap junctions
Table 1. Mechanism of
Membrane transport
|
Passive
Diffusion (Transcellular
Route) |
The
drug substance moves from an area of high to lower concentration without the
use of any energy. |
Depends
on partition coefficient. If k is greater than 5 easy permeation. Ficks
law of diffusion describes concentration gradient. |
|
Carrier Mediated
Transport Facilitated
Diffusion Active
Transport |
In
this mechanism the solute molecules get bind to carrier either reversibly or
via noncovalent bond to get transported. No
energy is required as concentration gradient is the driving force. The
driving force is against the concentration gradient called uphill transport
as energy required. |
Metabolic
poisons affect them. Metabolic
poisons affect energy production so does not affect them. Metabolic
poison blocks them. |
|
Pore
Transport (Paracellular
Transport) |
Absorption
of water soluble and low molecular size drugs through small pores or narrow
channels filled with water |
Osmotic
or Hydrostatic force is the driving factor. Depend
on molecular size should be low. |
|
Ion
pair Formation (Paracellular
Transport) |
When
ionized drug binds to an oppositely charged ion where overall charge is
neutral. |
Neutral drug complex diffusion is easy. |
|
Endocytosis Phagocytosis Pinocytosis Transcytosis |
Extracellular
material is engulfed by the cell using the part of cell membrane forming the
vesicle. It
is adsorptive uptake of solid particles. It
is uptake of fluid solute. Endocytosis
vesicle is transferred from one extracellular compartment to another. |
Formation
of phago-lysosome. Vesicle
formation Transport
of vesicle |
Factors Affecting Drug Absorption
Physicochemical
Factors
Table 2. Physicochemical
Factors that Affect the Rate and Extent of Absorption
|
Drug
Solubility and Dissolution Rate |
Drug
should be permeable through the cellular membranes and should be soluble for
complete drug absorption. |
|
Particle
size |
Smaller
particle size results in greater surface area that increases the dissolution
rate and as a result absorption is increased. |
|
Polymorphism
and Amorphism |
As
a drug can exist in more than one form having different physical properties
that affect dissolution rate and absorption. |
|
Pseudo
polymorphism |
Hydrates
and Solvates are present that have entrapped solvents. Solvates
are more soluble and have increased absorption. |
|
Salt
of Drug |
It
may increase or decrease absorption depending on drug. |
|
Lipophilicity
of Drug |
It
depends on oil-water partition coefficient the increase in value indicates
increase in percentage drug absorbed. |
|
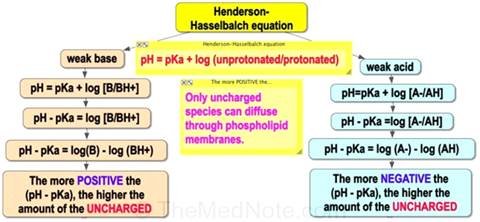
pKa
of Drug and Gastrointestinal pH |
Henderson-Hassel
Bach equation. |
|
Stability
of Drug |
Orally
used drugs may degrade when administered due to first pass effect. |
Figure 1
Henderson-Hasselbalch Equation
Physical Factors
Route of Administration
There are different routes of administration that effect the absorption
of drug. The main routes are oral, parenteral, sublingual, Topical route,
Enteral routes.
Bioavailability
Bioavailability is a type of absorption in which the drug concentration
enters the circulation when administered
in body and act on the specific organ to produce the desired effect. In
the given diagram the bioavailability of intravenous administration is 100% as
it directly reaches the systemic circulation while that of oral is less as it
may encounter a number of processes as first pass metabolism.
Pharmaceutical Factors
Table
3. Pharmaceutical
Factors that Affect the Drug Absorption
|
Disintegration
Time |
Low
disintegration time is required for rapid absorption. It
depends on amount of binder and compression force. |
|
Dissolution
Time |
It
affects the drug absorption as to be absorbed a drug needs to solubilize in a
specific solvent. |
|
Manufacturing
variable |
Drug
dissolution is influenced by manufacturing processes. Wet
granulation but it has several limitations so is replace by direct
compression force and affects dissolution and absorption depending on drug. |
|
Pharmaceutical
ingredients Vehicle Diluent Binder Disintegrant Lubricant Suspending
Agent Coating |
As
the number of excipients increases the dissolution becomes complex. Miscible
vehicles cause rapid absorption. Hydrophilic
diluents impart absorption while hydrophobic diluents retard absorption. Hydrophilic
binders enhance dissolution while an increase in binder amount retards
absorption. Mostly
hydrophilic in nature as amount of disintegrant decreases bioavailability
lowers. Hydrophobic
in nature and inhibits dissolution and disintegration. The
dissolution rate depends on type of coating as dissolution of enteric coated
is least. |
|
Nature
of Dosage Form |
The
absorption depends directly on the type of dosage form as the bioavailability
of solution is highest and that of sustained release products is lowest. |
Other Factors
Table
4. Some Unpredictable
Factors that can Alter the Drug Absorption
|
Age |
High
stomach ph and less flow of blood in GIT in infants and in elder patients
gastric emptying time is altered and GIT blood flow is less so pattern of
absorption is altered. |
|
Gastric
Emptying Time (The
process by which food leaves stomach and enters duodenum) |
Rapid
Gastric Emptying Time required when drug is absorbed from distal parts of
intestine. Prolong time is required when drugs are absorbed from proximal
parts. |
|
Intestinal
Transit Time (The
time taken by food to travel from mouth to intestine) |
Delayed
Intestinal Transit is desirable for sustained release products, enteric
coated formulations, drugs dissolved from specific sites of intestine. |
|
Gastrointestinal
pH |
Drugs
absorption takes place in different parts of stomach depending on their
pH. |
|
Disease
State |
Gastric
Diseases as Achlorhydric patients have decreased drug absorption.
Cardiovascular diseases influence bioavailability of drug and result in
decrease drug absorption. |
|
Blood
Flow Through GIT |
Absorption
of polar molecules does not depend on blood flow, but absorption of lipid
soluble molecules depends on blood flow. |
|
GIT
Contents |
Food-food
Interactions affect the intestinal pH and solubility of drugs. Fluid Volume
when large causes better dissolution and better absorption. It can interact
with other GIT constituents as mucin a protective layer of polysaccharide
that react with drug streptomycin. |
|
Presystemic
Metabolism The
metabolism of the drug before it reaches the systemic circulation via the
eliminating organs e.g. liver. |
Luminal
Enzymes as pepsin, Lipases etc. result in degradation of food and effect
absorption. Gut wall Enzymes called mucosal enzymes as Alcohol dehydrogenase
that inactivates ethanol. Bacterial Enzymes and Hepatic enzymes also effect
absorption. |
Distribution
It is a process in which the drug moves reversibly from the blood to the
extracellular tissues and fluid and move back to blood. Driving force is the
concentration gradient indicating that it’s a passive process.
Distribution of a
drug is a useful parameter as it changes the amount of the drug available at
the site of action altering the pharmacological action of the drug.
Drug Distribution in Different Body
Compartments
Table 5: Body Compartments Distribution and some Drugs having Affinity for Specific
Compartments
|
Body
compartments |
Types
of drugs |
|
Total
body water |
Small,
hydrophilic alcohol and antipyrine |
|
Extracellular
space |
Large
hydrophilic mannitol |
|
Intravascular
space |
Very
large, largely protein bound, heparin |
|
Body
fat |
Highly
hydrophobic DDT and thiopentone |
|
Bones
|
Fluoride
and lead |
Volume of Distribution
The volume of the
fluid in which the drug is distributed after administration. The distribution of the drug across the
extracellular tissues and blood stream (plasma) can be quantified using the
apparent volume of distribution.
Vd
= Dose of drug given (Q)
----------------------------------------
Drug plasma concentration (Cp)
Apparent
Volume of Distribution of some Drugs
Table 6: Some Common
Drug’s Apparent VD
|
Drug |
Liter/KG |
Liter/70 KG |
|
Choloroguine |
94-250 |
94-250 |
|
Nortriptyline |
211 |
500 |
|
Digoxin |
7 |
500 |
|
Lidocaine |
1.7 |
120 |
|
Theophylline |
0.5 |
35 |
Special compartments for drug distribution
Table
7. Special Compartments-Based distribution of some Drugs
|
Reservoirs |
Details |
Example |
|
Cellular
|
Skeletal
muscles, heart Thyroid
Liver |
Digoxin Iodine Chloroquine
|
|
Fats
|
Highly
lipid soluble drugs |
Thiopentone
sodium |
|
Transcellular
|
Aqueous
humour Joint
fluid |
Chloramphenicol Ampicillin
|
|
Bones
|
- |
Tetracyclines,
calcium |
Physiological Barriers to Drug Distribution
BBB
(Blood brain barrier)
Firmly joined by
tight junctions, then capillary endothelium. Few pores between cells. (Limits
passage of drugs to brain). Intracellular or transcellular transport is the
principle route for drug penetration into brain.
Lipid soluble substances diffuse across brain
capillaries based on lipid/water coefficients. Partially ionized, and moderate
lipid soluble drugs cross slowly. Restricts small polar molecules and
macromolecules.
Figure 3
Drug Permeability
Blood CSF Barrier
Choroid plexus is formed by third, fourth and lateral ventricle. Tight junctions are present in between the choroid cells although the open junctions are present in the capillary cells lining the choroid plexus but still only the lipophilic and non-ionized drugs are able to cross it.
It is not connected with tight junction. Penicillin belonging to less lipophilic category can only cross the BBB when administered via the intrathecal route and then they can treat the diseases of the brain.
Figure 4
Blood-CSF Barrier
Placental
Barrier
Most lipid-soluble
drugs readily move from mother to fetus (Diazepam), whereas water-soluble drugs
move more slowly. Highly polar or ionized drugs are more limited (Heparin). Has
several placental transporters that facilitate or block transfer? The Placenta
is not an effective barrier in protecting a fetus. Many Drugs can cross
placenta and result in therapeutic, toxic, or teratogenic effects.
Table
8. Drugs
that can Cross Placental Barrier
|
Drugs |
Effect
on fetus |
|
Methotrexate
|
Hydrocephalus: neural tube defects |
|
Phenytoin |
Cleft
lip and palate: cardiac defects |
|
Aminoglycosides |
Cochlear
and vestibular damage |
|
Carbimazole
|
Goiter:
hypothyroidism |
|
Warfarin
|
Nasal
hypoplasia; epiphyseal calcification |
Metabolism
Biotransformation
means chemical alteration of the drug from one form into another to make the
nonpolar drug to polar in order to excrete it from the body.
Sites of
Metabolism
·
Liver is the main site of metabolism
·
Kidney, lungs, plasma, intestine and Skin also
contribute to the metabolism of drugs.
Biotransformation of Drug
It basically converts
lipid soluble drugs to water soluble drugs
Consequences
of Biotransformation
·
Active drug to inactive metabolite
·
Active drug to active metabolite
·
Inactive drug to active metabolite
Chemical
Pathways of Biotransformation
A.
Non synthetic / Functionalization/ Phase I
B.
Synthetic / Conjugation/ Phase II
Phase I
Reactions
Oxidation, Reduction,
Hydrolysis, Cyclization & Decyclization
Phase II
Reactions
Glucuronide
conjugation, Acetylation, Methylation, Sulfate conjugation, Glycine
conjugation, Glutathione conjugation & Ribonucleotide / Ribonucleoside
synthesis
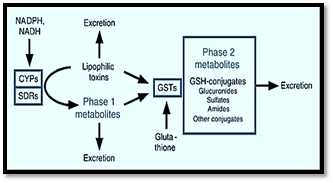
Figure 5
Drug Metabolism
Phase I Reactions
Oxidation
Oxidation is the oxygen being added or by the loss of the electrons. It can make some unstable intermediates for example quinones and epoxides.
Reduction
It is the gain of electrons, oxygen being removed
a) Microsomal reduction
b) Non-microsomal reduction
c) KETO Reduction
d) AZO Reduction
Hydrolysis
Water is added to breakdown the drug substance. It is of two types.
a) Microsomal hydrolysis
b) Non microsomal hydrolysis
Cyclization
A process in which a straight chain compound is transformed to a closed ring type structure.
Decyclization
A process in which the closed ring structure of a drug substance is transformed to open structure.
Phase II Reactions
Conjugation
Drug or metabolite of phase 1 binds with endogenous substance produced by either the proteins or the carbohydrates. Functional groups of these two are joined by the covalent bonds.
Conjugation with Glucuronic Acid
Carboxylic acid containing drugs are eliminated and metabolized significantly through this route.
Figure 6
Glucuronic Acid Conjugation
Acetylation
The process of introducing an acetyl group as a substitution for hydrogen atom is termed as acetylation.
Sulphate Conjugation
It is a process in which endogenous and exogenous are metabolically conjugated with sulphate(-SO3?).
Glycine Conjugation
In order to assist the excretion of the substances via the urinary route this process increases the solubility of organic acids in water. For example: Benzoic acid
Glutathione Conjugation
Glutathione combines with toxic substances and converts them into mercaptates that are water soluble. Acetaminophen and nicotine are detoxified very effectively via this pathway. Drug groups-Epoxide, Quinone
Methylation
The addition of methyl group to DNA molecule that tends to change its activity.
Ribonucleotide /Ribonucleoside Synthesis
Action of Purine & Pyrimidine antimetabolites (6 Mercaptopurine)
Excretion
Excretion is a process through which drugs substances are irreversibly transferred from the inside to the outside of the body.
Organs Involved in Excretion
Kidneys, lungs, saliva, skin, intestine as well as biliary system.
Types of Excretion
There are two types of the excretion broadly
? Renal excretion
? Non-Renal excretion
1. Salivary excretion
2. Mammary excretion
3. Dermal excretion
4. Biliary excretion
5. Pulmonary excretion
Renal Excretion
Most water soluble as well as non-volatile drugs are excreted primarily from the kidney. The urinary excretion of a drug is determined by three major processes
• Filtration via Glomerulus
• Active Tubular secretion
• Tubular reabsorption
Figure 7
Drug Excretion
Glomerular Filtration
High degree
filtration of the fluid is achieved through pores present in the capillary wall
of the glomerulus and it resist the flow of substances of high Mr. through it.
Plasma proteins are prevented through the selective filtration required to
maintain the volume of the plasma for example albumin and globulin. Shape, charge and molecular weight affect
the filtration of large molecules through the glomerulus. Until these
requirements are fulfilled, unbound drugs continue to be filtered through the
glomerulus. 20 to 40 angstrom compounds are efficiently filtered through
glomerulus. Glomerular filtration rate is usually ml per minute.
Active
Tubular Secretion
Many drugs that are
not filtered across the glomerulus tends to be secreted by the active secretion
from the blood into the kidney tubules. It is energy dependant process that
carries the substances against their concentration gradient using carries or transporters.
Table
7. Drugs Transported by
Anionic and Cationic Transporters
|
Organic
Anion Transport |
Organic
Cation Transport |
|
Acetazolamide |
Acetylcholine |
|
Bile
salts |
Atropine |
|
Hydrochlorothiazide |
Cimetidine |
|
Furosemide |
Dopamine |
|
Indomethacin |
Epinephrine |
|
Penicillin
G |
Morphine |
|
Prostaglandins |
Neostigmine |
|
Salicylate |
Quinine |
Active
Tubular Reabsorption
Some drugs depending
upon their ionization at the ph of the urine as well as their lipophilic
character are reabsorbed by the passive diffusion after being filtered by the
glomerulus. Therefore, the lipophilic drugs are about 99% reabsorbed from the
kidney tubules and hydrophilic drugs being soluble in urine and highly ionized
are eliminated via urine. Reabsorption via active transport is important for
the ions, amino acids, and glucose because they are endogenous substances
required by the body.
Biliary
Excretion
Hepatocytes secrete the bile juice at the rate of to
5ml per minute and it is essential for the breakdown of fats and subsequently
their digestion. Excretion depends upon
the polarity of the substance and metabolites being more polar are secreted
more than their parent drug. MW > 300 means large molecules are excreted
through the bile juice. Some drug substances mostly glucuronides are
metabolized by the hydrolysis done by the intestinal bacteria into the parent
compound which undergoes enterohepatic circulation.
Table 8. Drugs that
Undergo Enterohepatic Recirculation
|
Adriamycin |
Methadone |
|
Amphetamine |
Metronidazole |
|
Chlordecone |
Morphine |
|
1,25-Dihydroxyvitamin
D3 |
Phenytoin |
|
Estradiol |
Polar
Glucuronic Acid Conjugates |
|
Indomethacin |
Polar
Sulfate Conjugates |
|
\Mestranol |
Sulindac |
Drug’s long persistence in the body partly depends
upon enterohepatic cycling. Orally administered activated charcoal and/or anion
exchange resins have been used clinically to interfere enterohepatic cycling
and trap drugs in the gastrointestinal tract.
Pulmonary
Excretion
Gases and other
volatile substances are excreted by lungs, irrespective to their lipid
solubility. Alveolar transfer of the
gas/vapor mainly depends on its partial pressure in the blood. E.g.: Alcohol,
general anaesthetic etc.
Excretion
in Other Body Fluids
Saliva
Un-ionized
lipid-soluble form of the drugs are excreted by the passive means. Substances excreted into saliva are usually
swallowed so their fate resembles as that of orally administered. E.g.:
caffeine, metronidazole, alcohol etc.
Milk
Lactic secretions are
mainly present in milk so rich in fats and proteins with pH 7.0 0.5 to 1 litre
of the milk is
secreted in lactating mothers. Low-molecular
weight un-ionized water-soluble drugs will diffuse by passive transport across
the mammary epithelium and transfer into milk.
Conclusion
In conclusion, an overview of the pharmacokinetics is discussed. The basic parameters of pharmacokinetics are discussed to give an insight into the appropriate applications of ADME properties. In this review article important ADME factors are discussed that wholly described the concept of pharmacokinetics affecting the body as well as determining the safety and efficacy of a particular drug candidate. Knowledge about pharmacokinetic parameters have always emerged as important for providing optimal pharmaceutical care.
References
- Eason, C. T., Bonner, F. W., & Parke, D. V. (1990). The importance of pharmacokinetic and receptor studies in drug safety evaluation. Regulatory Toxicology and Pharmacology, 11(3), 288-307.
- Foti, R. S. et al (2015).
- Danhof, M. (2015). Kinetics of drug action in disease states: towards physiology-based pharmacodynamic (PBPD) models. J. Pharmacokinet. Pharmacodyn. 42, 447- 462
- Jones, H. M., Mayawala, K. & Poulin, P. (2013). Dose selection based on physiologically based pharmacokinetic (PBPK) approaches. AAPS J. 15, 377- 387.
- Morgan, P. et al (2012). Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving phase II survival. Drug Discov. Today. 17, 419- 424.
- Rowland M, Benet, L. Z., & Graham, G. G. (1973). Clearance concepts in pharmacokinetics. J Pharmacokinet Biopharm. 1(2):123-36.
- Rowland, M., Lesko, L. J. & Rostami-Hodjegan. (2015). A. Physiologically based pharmacokinetics is impacting drug development and regulatory decision making. CPT Pharmacometrics Syst. Pharmacol. 4, 313-315.
Cite this article
-
APA : Naseem, U., Iqbal, F., & Shahnaz, G. (2016). A Comprehensive Insight on Pharmacokinetics. Global Drug Design & Development Review, I(I), 27-37. https://doi.org/10.31703/gdddr.2016(I-I).04
-
CHICAGO : Naseem, Urooj, Fatima Iqbal, and Gul Shahnaz. 2016. "A Comprehensive Insight on Pharmacokinetics." Global Drug Design & Development Review, I (I): 27-37 doi: 10.31703/gdddr.2016(I-I).04
-
HARVARD : NASEEM, U., IQBAL, F. & SHAHNAZ, G. 2016. A Comprehensive Insight on Pharmacokinetics. Global Drug Design & Development Review, I, 27-37.
-
MHRA : Naseem, Urooj, Fatima Iqbal, and Gul Shahnaz. 2016. "A Comprehensive Insight on Pharmacokinetics." Global Drug Design & Development Review, I: 27-37
-
MLA : Naseem, Urooj, Fatima Iqbal, and Gul Shahnaz. "A Comprehensive Insight on Pharmacokinetics." Global Drug Design & Development Review, I.I (2016): 27-37 Print.
-
OXFORD : Naseem, Urooj, Iqbal, Fatima, and Shahnaz, Gul (2016), "A Comprehensive Insight on Pharmacokinetics", Global Drug Design & Development Review, I (I), 27-37
-
TURABIAN : Naseem, Urooj, Fatima Iqbal, and Gul Shahnaz. "A Comprehensive Insight on Pharmacokinetics." Global Drug Design & Development Review I, no. I (2016): 27-37. https://doi.org/10.31703/gdddr.2016(I-I).04